HVACR Fundamentals 101
101-2: Heat
Page 4:
Enthalpy is defined in the American Heritage Dictionary as a thermodynamic function of a system, equivalent to the sum of the internal energy of the system plus the product of its volume multiplied by the pressure exerted on it by its surroundings. (From the Greek enthalpien - to heat)
In the HVACR world, enthalpy is more simply defined as the total heat content of a substance at a certain base temperature, often 32° F, the point at which water changes between solid and liquid states. In the case of refrigerants, enthalpy is often measured at -40° F.
The following is an example of the enthalpy of water:
 |
Click to enlarge |
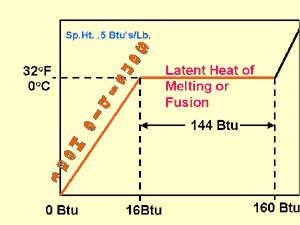
The lower left corner of the chart represents 0° F and 0 Btus. (Enthalpy is initially measured at 0°F for this representation of H2O.)
From 0° to 32° F water is in its solid state (ice). The specific heat of ice is .5 Btu, so, to raise one pound of ice from 0° to 32° requires 16 Btus. (.5 Btus X 32° F X 1 pound of H2O) The same pound of ice at 32° F requires an additional 144 Btus latent heat to achieve the physical change of state from solid to liquid — water.
The textbook, in referring to Fig. 2-1, mentions the strong bond between molecules in the solid state. It takes energy to loosen these bonds and thereby create a liquid state. As noted, changing one pound of 32° F ice to one pound of 32° F water takes 144 Btus. Note that there is no change in the temperature of the substance. The 144 Btus is the latent heat of melting or latent heat of fusion, depending on whether heat is added or removed
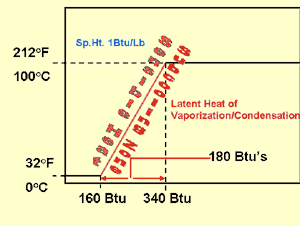
To drive the pound of water from 32° F up to 212° F, just before it boils, requires 180 Btus. Again, the specific heat of water is 1 Btu. The difference between 32 to 212° F is 180° of sensible heat.
Between the 32° F liquid and 212° F liquid water would be considered a sub-cooled condition.
 |
Click to enlarge |
At 212° F, liquid water is ready to change state to steam (vapor). The latent heat required to change one pound of 212° F water to one pound of 212° F steam is 970 Btus, an incredible amount of energy without a change in temperature. This is the energy required to break the bonds of liquid molecules. When the molecules separate, they fly into space as individual molecules full of energy. This is the latent heat of vaporization or latent heat of condensation, depending on whether you are adding or removing heat.
 |
Click to enlarge |
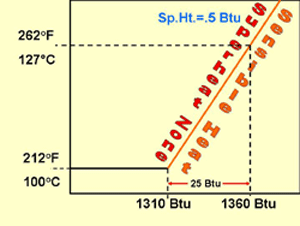
At this point, the one pound of 212° F steam is at the very beginning of the superheated vapor incline. The specific heat of steam is .5 Btu. With the addition of .5 Btus, the pound of steam will increase in temperature 1° F. This is referred to as superheat, which is a sensible heat because you can measure it with a thermometer.
Using the information above and following the chart, calculate the amount of heat necessary to raise one pound of ice from 12° F to 232° F steam.
Ice has a specific heat (review the definition) of .5 Btu. An increase from 12°F to 32°F — 20°F — requires 10 Btus of heat. (20°F X .5 Btus Sp. Ht. X 1 pound)
The latent heat of melting 1 pound of 32° F ice to 32° F water (noted above) is 144 Btus.
The specific heat of water is 1 Btu. The increase from 32° F water to 212° F water is a sensible heat of 180° F, and requires 180 Btus (180°F X 1 Btu Sp. Ht. X 1 pound).
The latent heat of vaporization required to change one pound of 212° F water to 212° F steam is 970 Btus.
The specific heat of steam is .5 Btu. The increase from 212° F steam to 232° F steam is 20° F. 20° F X .5 Btu Sp. Ht. X 1 pound = 10 Btus
Question: How much heat did it take? Answer: 1314 Btus (Add up the RED Btus).
You will be responsible for solving this type of problem. If the calculation isn’t for water, it may be for some other substance.
Refresher - Addition of Whole Numbers
Refresher - Multiplication of Decimals
Answer the following question: How many Btus are needed to transform one pound of ice at 30° F into one pound of steam at 214° F?Copyright © Blue C LLC. All rights reserved.